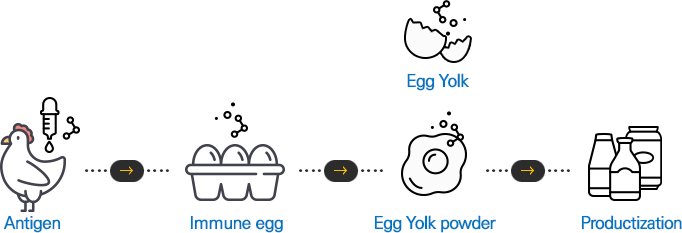
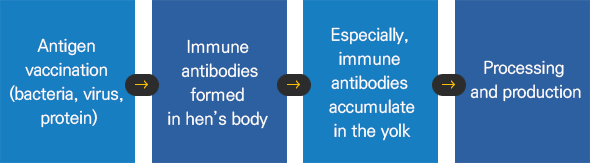
For you to eat safe food,
ADBIOTECH’s Central Research Institute strives to research technology and develop products.
ADBIOTECH’s Central Research Institute is an affiliated research institute certified by the Korea Industrial Technology Association and was established to research and develop pharmaceuticals, oral antibody products for livestock and fisheries, and food additives using egg yolk antibody (IgY) technology. Through industry-university collaboration, this research institute is leading national research projects for new and diverse products with the goal of “safe food for us”
for medicine, livestock, and fishery
Yolk antibody technology
01
for specific diseases in humans and livestock
Natural substances
02
to enhance immunity
Supplements
03